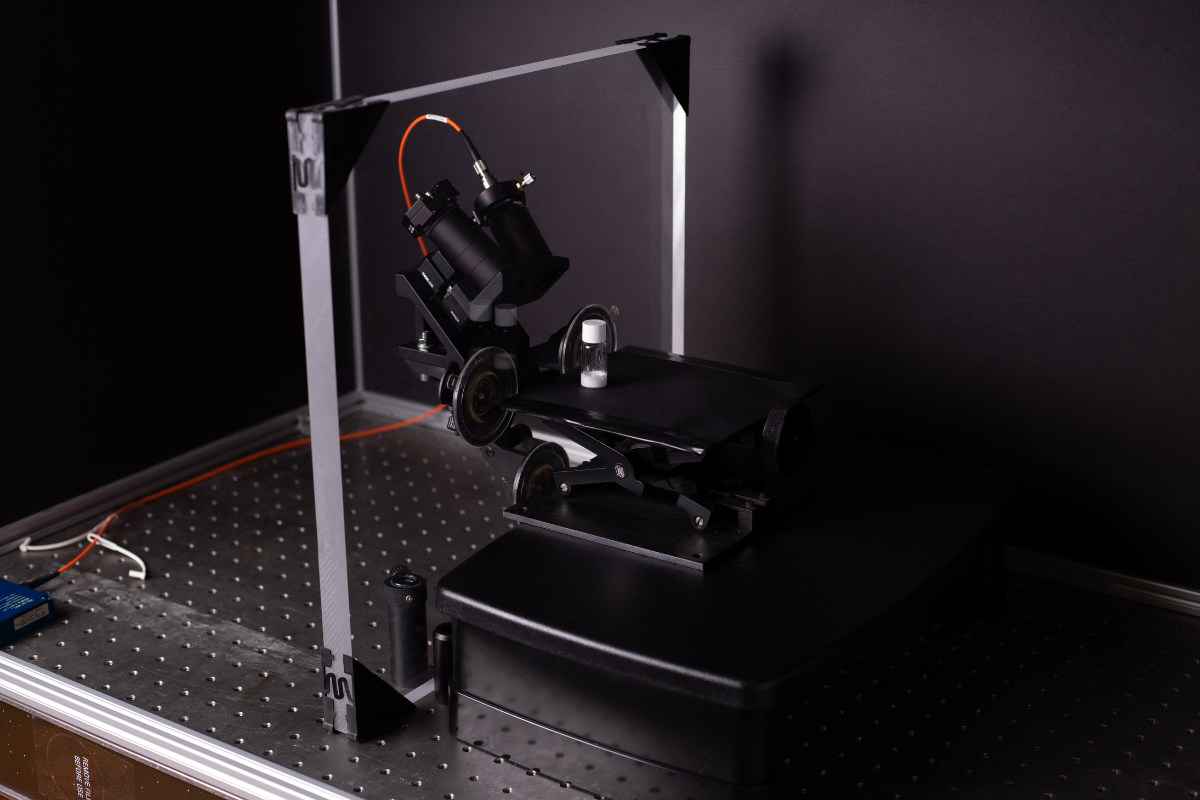
When the Digital_Lyo project began in 2023, it aimed to tackle one of the most complex and energy-intensive stages of biopharmaceutical manufacturing: freeze-drying (lyophilisation). Two years later, the project has been completed. The results are shaping how biologic medicines of the future will be developed and manufactured.
Why Freeze-Drying Matters
Biologics include advanced medicines such as vaccines and therapeutic proteins. They represent one of the fastest-growing areas in healthcare. Yet, these drugs are fragile and require strict temperature control to remain stable. As a result, manufacturers use continuous cold-chain systems that span from production to the patient, thereby increasing environmental impact.
Fortunately, freeze-drying reduces this challenge by removing water under vacuum to create a stable, dry product. The dried product can therefore be stored safely at room temperature. However, lyophilisation remains slow, energy-intensive, and challenging to control. Existing methods typically check product quality only after the process is complete. Consequently, one error can result in wasted time, energy, and an entire batch.
The Digital_Lyo Vision
Digital_Lyo was funded through the UK Government’s Digitalisation and Automation of Medicines R&D and Manufacture competition, delivered by Innovate UK. Its goal was simple but ambitious: to bring advanced process-monitoring tools and digital-twin modelling to freeze-drying, creating a smarter, more sustainable process that supports both product quality and net-zero targets.
The project brought together a unique consortium:
-
IS-Instruments – Raman spectroscopy specialists developing a new 1064 nm system to capture real-time chemical data during lyophilisation.
-
LyosenZ – developing through-vial impedance spectroscopy (TVIS) to monitor electrical properties of samples throughout the process.
-
De Montfort University (LyoGroup) – providing analytical routines and mapping TVIS data to process parameters.
-
Qrometric Ltd – experts in humidity and vapour-pressure metrology.
-
Siemens – contributing its digital-twin technology for modelling and predictive control.
-
AstraZeneca plc – leading the industrial evaluation and supplying real-world assets for testing.
-
MHRA – advising on formulation standards and validation.
ISI’s Contribution: Seeing Inside the Process
Raman analysis detects subtle molecular changes within a sample in real time. It reveals how materials transform during freezing, primary drying, and secondary drying. However, using Raman in lyophilisation introduces significant challenges. Measurements must pass through thick acrylic walls and borosilicate glass vials, creating a difficult environment for achieving clear optical signals. Additionally, biologic samples also fluoresce strongly, thereby masking the Raman signal.
ISI solved these issues by developing a new 1064 nm Raman system to overcome these problems. This wavelength reduces fluorescence, producing a clearer spectral signature. The team integrated the Raman system with LyosenZ’s impedance spectroscopy and Qrometric’s humidity sensors.
Together, they formed a multi-PAT (Process Analytical Technology) platform.
As a result, Digital Lyo captured combined, real-time measurements of molecular, electrical, and environmental data throughout the freeze-drying process.
Data That Drives Smarter Decisions
Researchers built a digital twin environment in collaboration with Siemens and De Montfort University, and then fed the collected data into it. This collaboration enabled teams to simulate and optimise each stage of the freeze-drying process. They evaluated heat and mass-transfer parameters to predict how products behave in different dryers and scales. Consequently, the digital twin supports accurate process transfer and efficient industrial scale-up.
Using this data-driven model, the teams could adjust the process dynamically in real-time. It shortens development cycles and reduces overall energy use. In turn, manufacturers gain faster product development, fewer failures, and more consistent product quality
Real-World Impact
The potential impact of the Digital_Lyo technologies is substantial:
✅ CO₂ reduction: An estimated 700 tonnes of CO₂ savings per new product through more efficient process development and up to 300 tonnes per year per freeze-dryer during operation.
✅ Improved reliability: Better data means faster troubleshooting and fewer failed batches.
✅ Sustainable supply chains: Shorter cycles and improved yield help reduce waste and support more resilient medicine supply networks.
The project also explored future-ready technologies such as Peltier-based continuous freeze-drying systems, which could replace traditional high-GWP refrigerants and offer step-change reductions in energy demand.
From Research to Reality
For IS-Instruments, Digital Lyo achieved far more than a proof of concept. The project produced a new Raman system configuration validated in demanding pharmaceutical environments. Building on this success, the company is now preparing to launch the system commercially. It will offer both direct sales and service-based options to reach process-development labs and manufacturers.
Meanwhile, DMU has been awarded funding to establish a Centre of Excellence to further develop multi-PAT and digital-twin solutions. Ultimately, this will ensure the UK remains at the forefront of pharmaceutical innovation.
What Comes Next
As biologic medicines become increasingly vital to modern healthcare, the need for sustainable, high-precision manufacturing will only grow. By helping to make freeze-drying faster, smarter, and cleaner, this project has demonstrated how advanced optical technology can support both scientific progress and environmental sustainability.
Although the funded phase of Digital_Lyo has concluded, its legacy is only just beginning. IS-Instruments will continue to refine its Raman technologies for broader process-monitoring applications, spanning pharmaceuticals, advanced materials, energy, and environmental systems.