The InSPIREmed project – Integrated Spectroscopy and Photonics for Increased Productivity and Resource Efficiency in Medicines Manufacture – is a major UK initiative aimed at redefining how modern pharmaceuticals are developed and manufactured. Funded through the Innovate UK Sustainable Medicines Manufacturing Programme (SMMIP), the project brings together a consortium of world-leading organisations to develop a new generation of high-performance, integrated photonic Process Analytical Technology (PAT) systems.
These systems will support real-time monitoring and control of critical pharmaceutical operations, enabling cleaner, more energy-efficient, and more reliable manufacturing processes.
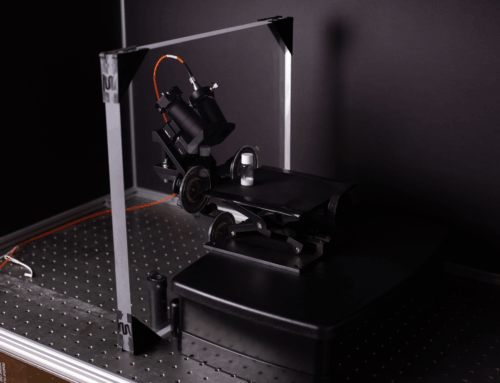
IS-Instruments (ISI) is a core technology partner in this programme, contributing state-of-the-art Deep-UV Raman spectroscopy solutions that provide the molecular sensitivity needed for next-generation sustainable bioprocessing.
Why InSPIREmed Matters
The pharmaceutical sector is under increasing pressure to improve the sustainability, resilience, and efficiency of its manufacturing processes. Many current methods are limited by:
-
Slow or infrequent offline analysis
-
Energy-intensive operations such as freeze-drying
-
Limited visibility of molecular changes in complex biological systems
-
A lack of integrated, real-time decision tools
-
Quality risks associated with batch-based processing
To address these challenges, the industry requires robust, high-resolution PAT tools capable of operating in demanding environments while delivering real-time, high-fidelity data.
InSPIREmed focuses specifically on integrating advanced photonics, including Raman, deep-UV Raman, mid-IR, and particle-scale optical sensing, with digital control and automation. By doing so, the project will create a unified analytical framework that supports:
-
Continuous verification of critical quality attributes (CQAs)
-
Reduced energy and solvent consumption
-
Faster process development and optimisation
-
Greater manufacturing resilience and product consistency
A World-Class Consortium
The strength of InSPIREmed lies in its multidisciplinary consortium, which includes major pharmaceutical manufacturers, leading photonics groups, national laboratories, regulatory bodies, and digital technology experts.
Key partners include:
Photonics & Measurement Specialists
-
IS-Instruments (ISI)
-
Sparta Biodiscovery
-
Dyneval
-
Particology
-
Vortex Optical Coatings
- Caledonian Photonics (subcontracted)
These groups develop the optical sensing and characterisation technologies that underpin InSPIREmed’s PAT framework.
Industry & Manufacturing Partners
-
Cytiva
-
CPI (Centre for Process Innovation)
- GSK (GlaxoSmithKline)
These organisations provide industrial bioprocess platforms for validation and deployment, ensuring the sensors developed are ready for real-world application.
Regulatory, Standards & Metrology Bodies
-
MHRA (Medicines and Healthcare products Regulatory Agency)
-
NPL (National Physical Laboratory)
-
BSI (British Standards Institution)
Their involvement ensures the analytical tools and data structures developed are compatible with future regulatory guidance and GMP expectations.
Digital & Academic Partners
-
University of Strathclyde
-
Optimal Industrial Automation
-
VisionMetric
They support advanced modelling, multimodal data fusion, control strategies, and the development of computational frameworks for automated decision-making.
Together, this consortium provides the whole chain from sensor development to deployment in regulated industrial settings.
ISI’s Role: High-Sensitivity Deep-UV Raman for Real-Time Bioprocess Monitoring
ISI’s contribution to InSPIREmed focuses on developing compact, high-sensitivity Deep-UV Raman (DUVRR) instrumentation for use in challenging bioprocess environments. Deep-UV Raman provides resonance enhancement of biomolecular signals, allowing ultra-sensitive detection of:
-
Protein conformation and structural integrity
-
Aggregation and degradation pathways
-
Excipient interactions
-
Subtle physicochemical changes during freeze-drying and perfusion processes
This level of sensitivity is essential where traditional NIR, MIR, or visible-range Raman systems struggle due to fluorescence interference or low signal strength.
Two key InSPIREmed application areas for ISI technology include:
1. Real-Time Monitoring of Freeze-Drying
Freeze-drying (lyophilisation) is one of the most energy-intensive stages in biopharmaceutical production, and current monitoring techniques often lack molecular resolution. ISI’s DUVRR system will allow:
-
Through-vial, non-invasive monitoring
-
Tracking of structural changes in proteins and excipients
-
Identification of endpoints and instability during drying
-
Optimisation to reduce cycle times and energy consumption
2. Monitoring Monoclonal Antibody Perfusion
Continuous perfusion processes require precise control of protein quality attributes. ISI’s DUVRR platform will support:
-
Inline structural characterisation of biomolecules
-
Early detection of aggregation or degradation
-
Real-time optimisation of nutrient feeds and harvest cycles
-
Improved yield with reduced water and energy use
Why ISI Was Selected: Technical Foundations from ATLAS-BIO and Digital_Lyo
ISI’s involvement in InSPIREmed builds directly on the technical successes of two previous programmes:
Digital_Lyo
During Digital_Lyo, ISI developed a robust through-vial Raman monitoring system capable of detecting molecular transitions during freeze-drying in real time. This proved that photonics could provide meaningful, inline molecular insight in low-temperature, reflective, and highly dynamic environments.
ATLAS-BIO
This programme demonstrated ISI’s ability to apply deep-UV Raman to complex biologics, including protein structural analysis, aggregation monitoring, and characterisation of cell-culture media.
Together, these projects established:
-
Proven capability in DUV Raman instrument development
-
Expertise in integrating photonics with automated control systems
-
Understanding of bioprocess-specific analytical challenges
-
Validated hardware ready for industrial progression
This track record positioned ISI as a natural contributor to InSPIREmed’s photonic PAT workstreams.
Shaping the Future of Sustainable Bioprocess Manufacturing
InSPIREmed represents a significant step forward for the UK’s medicines manufacturing sector. By uniting advanced photonic sensing, digital modelling, and real-time control, the project will enable:
-
More efficient biologics production
-
Shorter and cleaner freeze-drying cycles
-
Reduced energy and solvent usage
-
Improved product consistency and process reliability
-
Wider adoption of automated and continuous manufacturing approaches
ISI is proud to contribute its specialist Raman expertise to this ambitious programme and to work alongside a consortium committed to delivering the next generation of sustainable, intelligent biomanufacturing technologies.