Welcome to the world of IS-Instruments
Leading the way in the development of compact spectrographic and remote sensing technologies.
About IS-Instruments
Identifying the unknown so our clients can make informed decisions.
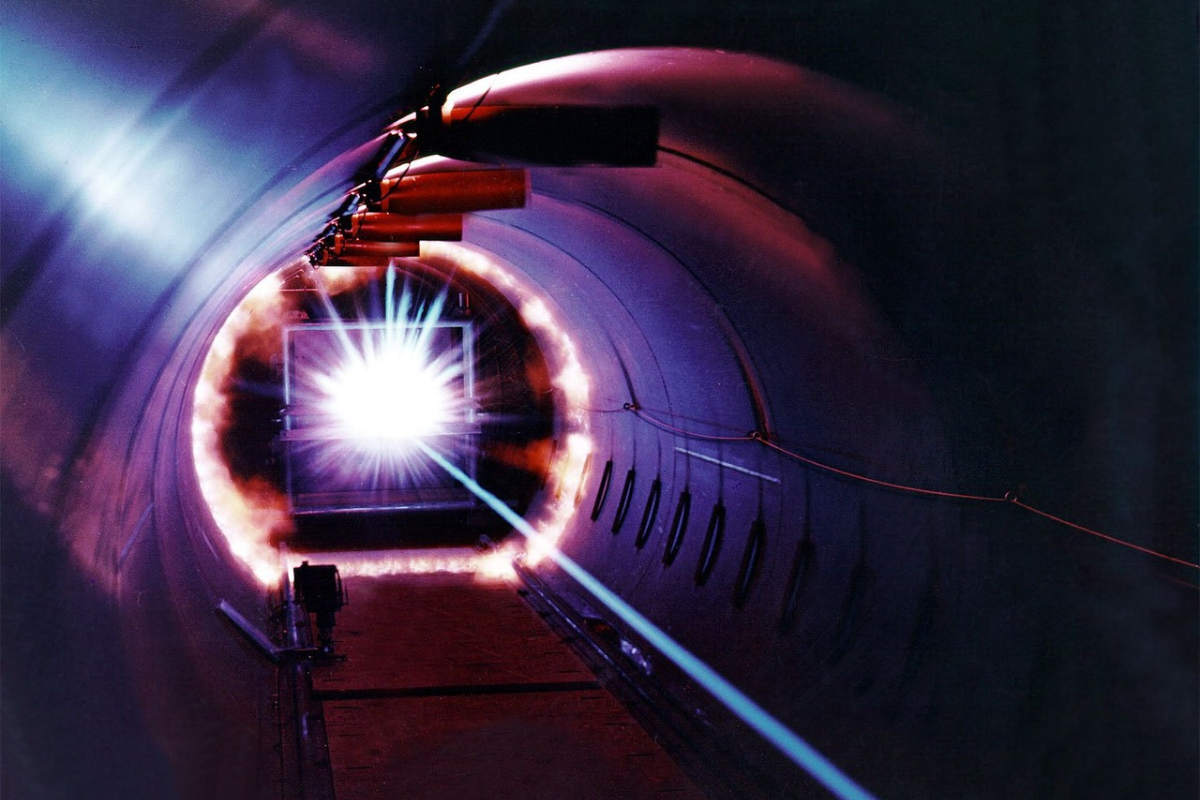
Our range of spectrometers, laser-based instrumentation and bespoke LIDAR solutions provides our clients with a new generation of highly-sensitive measuring and identification tools. Originally developed for use in space, our instruments are leading the way in identifying and classifying substances in extreme environments and finding new ways of enabling advances in biopharmaceutical processes.
Working in collaboration with industry and academia, we’re perpetually evolving our spectrometers to deliver innovative and practical solutions for some of the toughest challenges we’re facing today.
Our in-house team has the knowledge and experience to offer tailored solutions for clients with specific requirements. That ability sets us apart as leaders in the field of spectroscopy and spectrographic solutions.
So tell us – what are you trying to measure?
About our Spectrometers
Our spectrometers use a fibre optic rather than a slit to allow the laser to reach its target. The benefit of this is less light ‘wasted’, allowing our spectrometers to have a higher throughput/etendue than traditional instruments.
We have developed a deep UV instrument, ODIN, to which our base technology lends itself well, using a self-aligning diode laser that eliminates the requirement for air purging.
Also in development is a system using hollow-core microfibre capable of making gas Raman measurements detecting multiple gas species within an environment on-site.
Some of the industries we work with:
- Nuclear / Nuclear Decommissioning
- High-value Manufacturing & Process Industries
- Pharmaceutical
- Biopharmaceutical
- Oil & Gas
Our Products
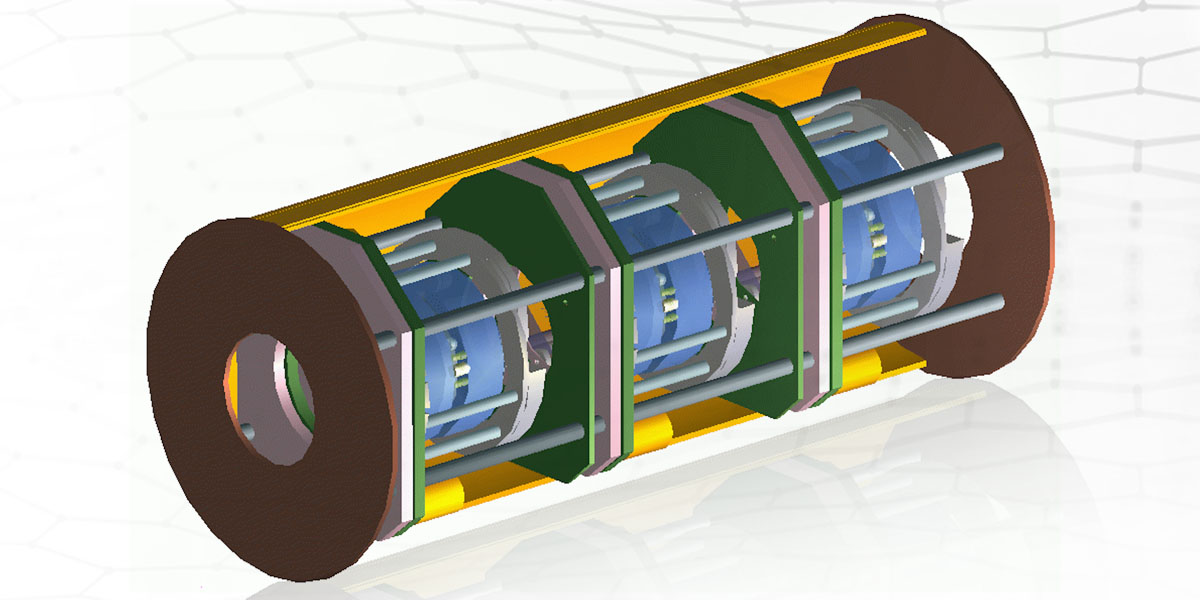
Our range of spectrometers and laser-based instrumentation includes the world’s highest throughput Raman spectrometer, Raman probes suitable for extreme environments, bespoke Fabry Perot and LIDAR solutions, as well as a WiFi-enabled miniature spectrometer.
Developing the tools for the future
Drawing on our space heritage and working with partners across academia and industry, we’re developing instrumentation to deliver remote sensing solutions for previously unsolved challenges.
So tell us – what are you trying to measure?
Discover the power of light with IS-Instruments
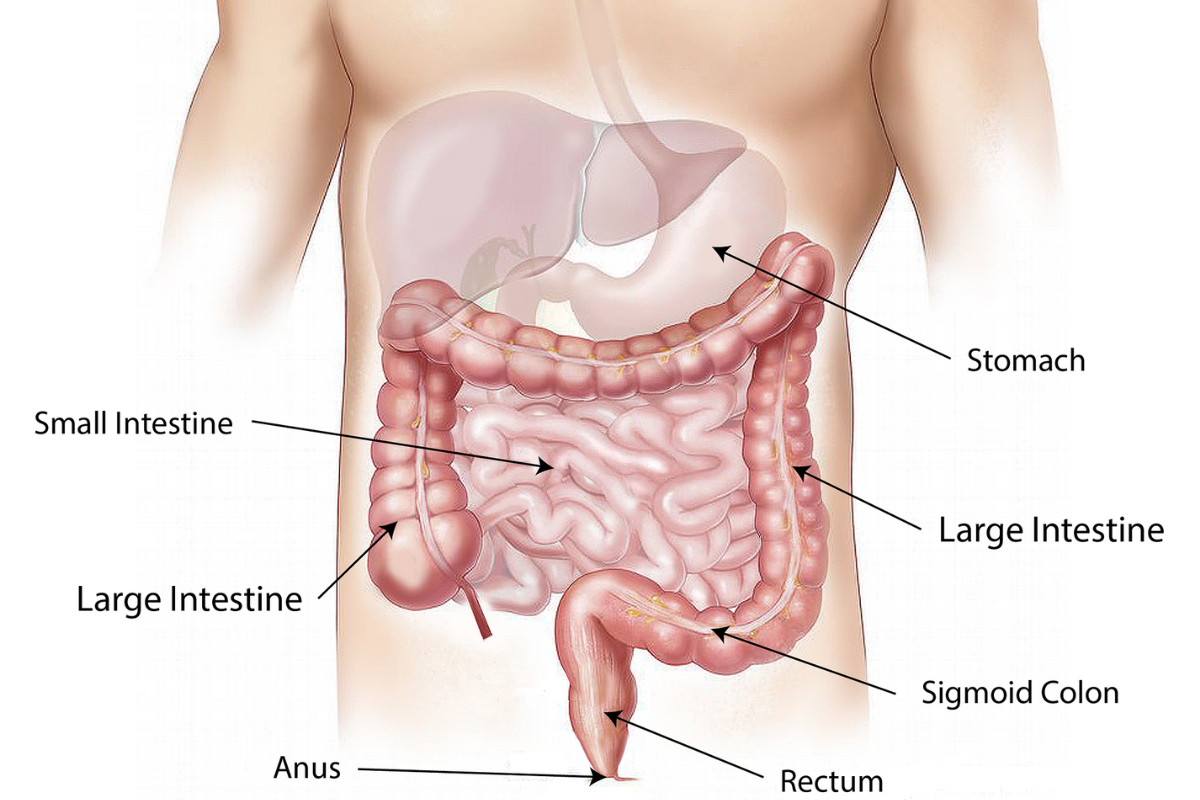
ISI Gets Into The Ring To Fight Bowel Cancer
IS-Instruments has won funding to develop new instrumentation exploiting advances in Raman spectroscopy to speed up bowel cancer diagnosis.
Bowel cancer […]
ISI Wins Funding to Develop Innovations In Freeze-Drying Technologies
ISI is part of a consortium that has won funding to develop in-situ process analytical technologies (PAT) for freeze-drying, a […]
Development Scientist Engineer Required To Join Our Team – We’re Hiring!
We’re hiring!
IS-Instruments is an SME based in Tonbridge, Kent, and we specialise in developing spectrometers, Raman systems and […]
Why Wavelength Matters in Spectroscopy
In its broadest sense, spectroscopy is used to investigate and measure spectra produced when a substance interacts with […]